Written by KJ Sakura
Mid-January of 2020, I had the great fortune of taking a once in a lifetime trip to Japan with leaders of the sake education industry. One of our itinerary days was devoted entirely to a place called NRIB, the National Research Institute of Brewing. It was founded in 1904 and is located in Higashi-Hiroshima in Hiroshima, Japan. In their own words, “We aim to contribute to the suitable and fair imposition of liquor taxes, the sound development of the alcoholic beverage industry, and the enhancement of public awareness regarding alcoholic beverages through the advanced analysis and evaluation of alcohol beverages, as well as fundamental and applied research and dissemination of information.”
Their analysis and evaluations are in cooperation with the National Tax Agency of Japan (who also sponsored our trip). It is a rare occasion to be able to spend an entire day in one of the most prominent beverage research facilities in the country. Some of their major operations include development of new analytical methods, testing for potentially harmful contamination and/or radioactive substances, and certification for exports such as Taiwan and the EU. They also conduct research regarding raw materials and components of beverages for taxation and labelling purposes, the study of microbial safety and improvement of fermentation processes, and the promotion of Japanese products such as sake, wine and shochu. They are in close collaboration with related organizations, including universities, other research institutes and the industry in general. Needless to say, this was a highly insightful day.
Some of NRIB’s major accomplishments include:
1909 – Development of a new method for yeast starter preparation (Sokujo)
1963 – Development of a practical method to discriminate sake yeast and wild yeast
1971 – Development of the breeding method of non-foaming sake yeast
1975 – Elucidation of the cause of deteriorated smell (oily smell) of shochu
2005 – Whole genome sequencing of Koji mold (Aspergillus oryzae) by a research consortium of industry, academia and government
2006 – Publication of the flavor wheel of sake
2011 – Operations for Fukushima Daiichi nuclear disaster: research and analysis to keep the safety and reliability of alcoholic beverages
After being warmly greeted by NRIB President Dr. Goto Nami, we had an hour long seminar on the sake making process followed by a short break and then an eye opening lecture by Dr. Akamatsu Fumikazu, the Senior Researcher of Quality and Evaluation Research Division. Various research and topics were explored which led to many insights connecting the worlds of sake and wine.

One of the research highlights was the comparison between components of sake compared to that of a typical white wine. As educators, most of us were aware of these differences, but to see the numbers on paper made it easier to visualize. Iron is commonly known to create issues in sake production, such as abnormal coloration and a deterioration of aroma and quality. Iron also disrupts yeast metabolism which can affect high risk sake fermentations more negatively than it would in wine production. Iron in sake clocks in at less than .02ppm, whereas white wine is on average 0.4mg/l (1ppm = 1mg/l) and red wine around 0.7mg/l. From sake to red wine, that is a 3500% increase, or 35 times the amount of iron without attributing any noticeable negative qualities.
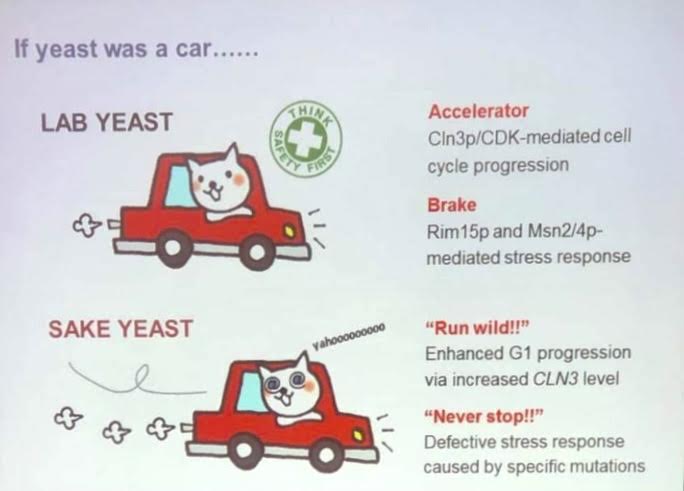
Alcohol and glucose readings were not too surprising considering sake yeasts are noted for fermenting to higher degrees of alcohol than wine yeasts. They are actually the same yeast strain known as Saccharomyces cerevisiae, but in sake production a mutant strain is used. This strain has no “off” button, and can ferment to over 22% alcohol. If it reaches above that amount, it is no longer legally sake! They presented this interesting information with a funny picture of a crazy cat in an out of control car. Sake yeast “runs wild” due to enhanced G1 progression via increased CLN3 levels and “never stops!!” due to a defective stress response caused by specific mutations in the strain (see photo below).
As for glucose, sake contains between 5-42g/l while white wine has 1-30g/l. The amount of glucose in both sake and white wine wasn’t too surprising, but what was intriguing was that the numbers were not that dissimilar. I always assumed sake had much more glucose than wine, but if you account for the entire spectrum of dry to sweet in which sake and wine both exist, it makes sense that the numbers aren’t too variable. White wine can actually reach much higher levels of sugar, for example, Auslese Rieslings from Germany can peak over 100g/l and the sweet sherry style Pedro Ximénez can reach heights of over 400g/l. But since yeasts are glucophilic (they selectively ferment glucose), these higher levels of remaining sugar are actually fructose.
Through the years I think I have casually confused glucose with glycerol; a byproduct of fermentation that creates texture and fullness. We weren’t presented any information about glycerol production in sake, but it’s these simple numbers that debunk misconceptions and strengthen common knowledge!
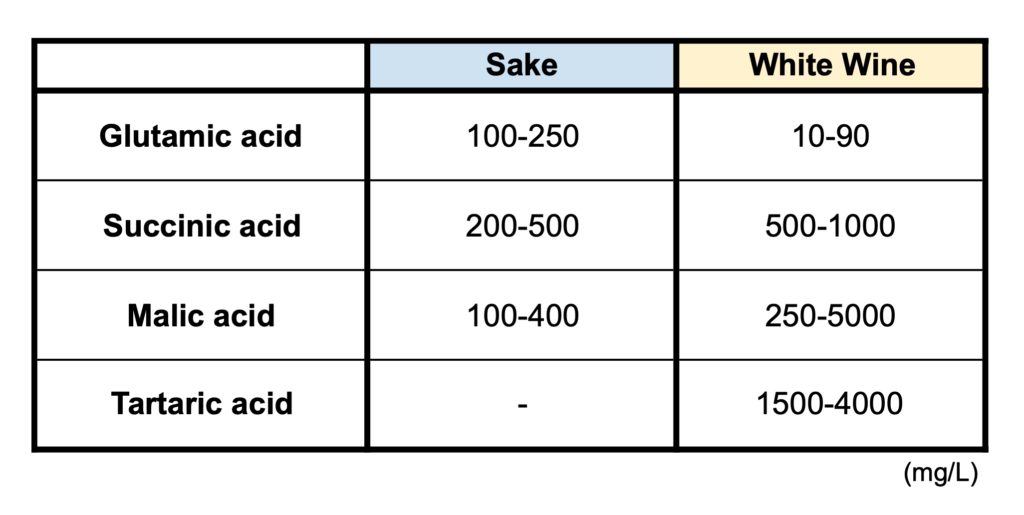
Acidity numbers were surprisingly thought-provoking. I always knew that sake has 1/5th the acidity that wine has, but I never realized that most of sake acid is coming from the yeast and in wine, more acidity comes from the raw materials. Of course this makes sense when research is clearly presented! Sake is also known for having a higher amount of amino acids, while wine is more driven by other types of acidity. Glutamic acid ranges between 100-250mg/l in sake and a much lower 10-90mg/l in white wine. This type of acid is weak in comparison to other types and is known for providing flavors of umami.
The following acids contribute more to the sensation of acidity in a beverage. Succinic acid in sake reaches 200-500mg/l in comparison to white wine at 500-1000mg/l. Malic acid (most commonly found in apples and pears) plays a smaller role in sake than wine, with 100-400mg/l existing in the prior versus 250-5000 mg/l in the latter. Lactic acid plays an important role in both wine and sake production and the levels depend on the type of shubo and style of sake produced. The same goes for wine. Most reds and some whites go through a process called malolactic fermentation (also known as MLF), where malic acids convert to lactic acid through lactic acid bacteria. Tartaric acid, found most commonly in grapes, but also citrus, bananas and tamarind, makes up the rest of wine’s elevated acidity. There is zero tartaric acid in sake and 1,500-4,000mg/l in a white wine’s composition.
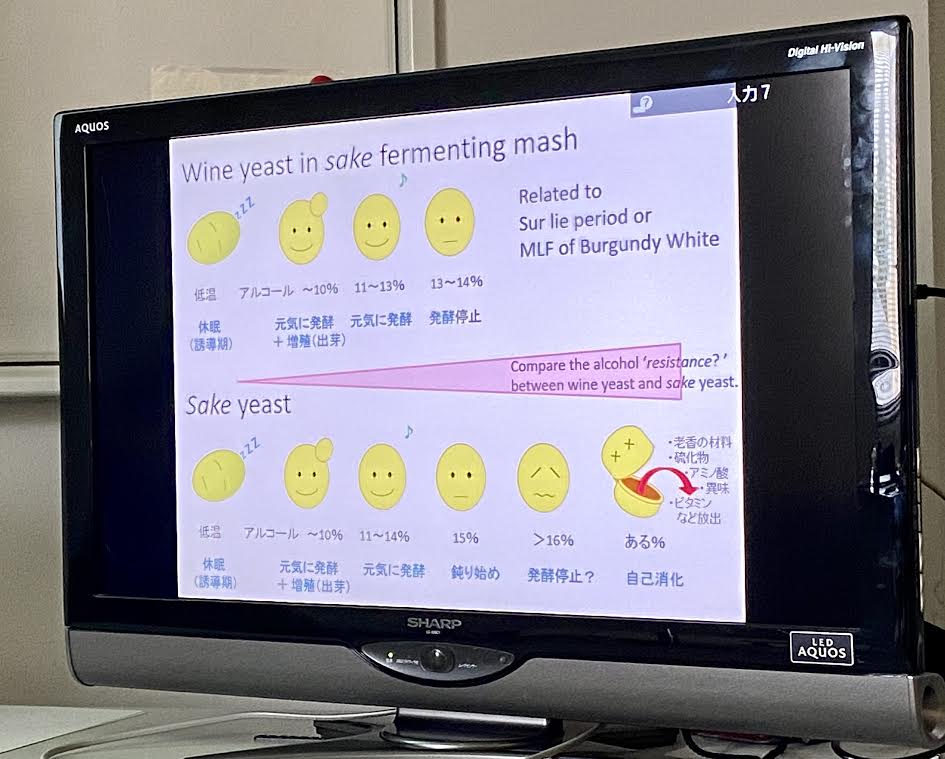
To elaborate more on sake yeast’s behavior, we were informed that these species show high ethanol productivity, but less tolerance to ethanol. The mutation which they share are genetic defects of their environmental stress responses. This is why they continue to ferment in high stress environments (high alcohol) while wine yeasts die at those levels.
Another fun revelation was the discovery of pink colored sake influenced by yeast! I had only come across one sake like this before my visit to Japan, which was a pink sparkling made by Takachiyo Shuzo. There is a red colorant that accumulates in this type of yeast and it was isolated for use in various sake styles. Isolation of yeast doesn’t stop there! Sake yeasts with deficient bubble forming abilities have been segregated to create non-foaming yeast strains. These were reported by NRIB and the Hiroshima Regional Taxation Bureau in 1916. By 1963, the demand for sake exceeded what was possible production-wise. Non-foaming yeasts allow brewers to make 25% more sake without increasing tank capacity. It is also less labour intensive and eliminates the need to clean foam off the tank walls or worry about foam overflow during active ferments. Due to the expression of the AWA1 gene in Kyokai K-7, they were able to create Kyokai K-701, which became the first non-foaming commercial yeast in 1971. Not all breweries utilize this type of yeast, claiming that the foam is an integral part of fermentation and can give clues to how it’s progressing just by observing it.
My favorite part of the day was learning about faults. For many years I had no idea that sake could have problems. I thought the only taint that plagued the category stemmed from hi-ochi spoilage bacteria in namazake without proper storage. I naively believed that pasteurized sake was somehow invincible. Boy was I wrong!
・Hine-ka
Sake experts who judge at a professional level know all about hine-ka. Hine-ka in English translates to “old stink” and has an undesirable aroma similar to tsukemono, or Japanese pickles. When amino acids decompose or a sake is left in higher than recommended temperatures, this fault can evolve. It consists of polysulfides, mainly DMTS, also known as dimethyl trisulfide. At high concentrations, another polysulfide called isovaleraldehyde can also contribute to this smell.
NRIB has done much research on DMTS. They found that yeast creates a precursor of DMTS (DMTS-P1). They also mentioned that leaving sake on the lees, which are dead yeast cells, helps contribute to the formation of DMTS. I found this quite perplexing, considering lees aging is very common in wine production. The technique plays a huge role in the old world regions of Muscadet, Champagne and Burgundy. Sur lie aging allows the yeast membrane to disintegrate while being in contact with a wine. The innards of the yeast dissolve into the liquid and can impart nutty and bread-like flavors. It can also enrich and stabilize a wine, as it does in red wine production. Coming from a wine-focused background, this concept seemed strange to me. How could lees aging create a fault in sake, yet be highly revered in the wine world? I asked Dr. Fumikazu this question and he said it came down to the culture of Japan and Europe being very different. In Japan, these aromas are considered faulty. In Europe, it’s the opposite. People have become accustomed to those flavors in those particular wines and it is part of their identity. Sur lie aging can create an off-putting leesy flavor if overdone, but the fact that this was more of a conceptual evolution in beverage perception over time versus a scientific one is utterly fascinating!
・Diacetyl
This famous compound is responsible for the buttery character in many Chardonnay wines from around the world. It can be a positive contribution to junmai-shu, but is largely considered an off-flavor. Malolactic fermentation happens in most red wines to enhance stability and in certain white wines where that buttery, creamy style is preferred. The lactic acid bacteria (LAB) which creates diacetyl is called Oenococcus oeni. Although this type of LAB is considered faulty in the sake world, bacterias such as Lactobacillus and Leuconostoc help in killing off wild yeast in the beginning of kimoto production. It is crazy to realize these subtle differences in microbiology between sake and wine production!
Maillard reactions occur in both wine and sake production. Most of us interact with Maillard reactions when caramelizing onions or toasting bread. It is not a single reaction, but a complex series of non-enzymatic browning. Heat induces these reactions but they can occur more slowly at room temperature. Amino acids and sugars combine and form compounds which are then further broken down into hundreds of flavors. It also involves amino-carbonyl reactions which create brown pigments called melanoidins. This is what gives an aging sake it’s color, ranging from bright yellow to amber over time. NRIB has taken the time to differentiate aromas developing from these types of reactions in contrast to hine-ka. Sotolon is the aromatic compound responsible for aged aromas such as honey, soy sauce, nuts and caramel. It can also smell like fenugreek or curry at higher concentrations. It is formed by condensation of acetaldehyde and a-ketobutyric acid which is considered part of the maillard reactions. Sotolon is the main culprit for distinctive aromas in oxidative wines such as Madeira, Vin Jaune and Sherry. They come off a bit more maple syrup, burnt sugar and walnut flavored in those wines and are linked to the oxidation of the beverage as well. In Champagne production, a disgorged bubbly that receives a dosage (added sugar) will eventually undergo maillard reactions and develop flavors of brioche, puff pastry and biscuit. A brut nature style sparkling that does not have added sugar will not go through this kind of flavor transformation.
An interesting study was done by a neighboring research group who found that the phenomenon of fishy odors and metallic smells when combining fish and wine comes not from tannin like previously assumed, but the higher concentration of iron in wine! Fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are prone to oxidation during processing. Lipid peroxide is produced and iron ions in wine act as a catalyst for the development of volatile carbonyl compounds. It has also been discovered that sulfites in wine exaggerate these reactions. If there is oil and lemon in a recipe, it will actually help reduce the perception of these adverse properties! As a wine professional, I am aware that even wines with no So2 additions contain sulfites created during the fermentation process. But I have to wonder… if more sulfites in a wine tend to embellish the interaction of iron with the fatty acids of fish, could this be why the Japanese market is so welcoming of the natural wine movement which eschews So2?
My last aha moment occurred while learning about shochu production. About a year and a half before this trip, I had judged shochu for the International Wine and Spirit Competition in London, England. I came across a multitude of samples that had beautiful, extravagant aromas of lychee and rose petals, similar to the grape gewürztraminer. We tasted everything blind, so I never really understood how certain shochu tasted like that. At NRIB, I realized that this aroma comes from vacuum distilled sweet potatoes at low pressure and temperature. The compound geraniol comes from the base mash which then evolves into citronellol, linalool and a-terpineol. Geraniol and linalool are terpenes found in both gewürztraminer and muscat grape varieties!

We later enjoyed an entertaining and informative introduction to Japanese history by Dr. Horie Shuji and then participated in fault and sake expert assessor trials in the afternoon. It’s amazing to think that after such a long day of learning, I still had to rehearse my ½ hour sake educator lecture for the next day! My greatest payoff from this incredible day of discovery was filling the blank spots in my comprehension. The exploration of microbiology and brewing techniques, in addition to the clarification between wine and sake production was more than any beverage professional could ask for. Thank you NRIB, the National Tax Agency and the Wine Spirit & Education Trust for a glorious day of research and knowledge!